Packaging Lines
Site and Corporate Repositories
Site and Corporate Repositories

We assess and recommend packaging line equipment requirements and layout designs to meet client requirements for unit level and aggregation serialisation.
Site and Corporate Repositories
Site and Corporate Repositories
Site and Corporate Repositories

We can upport/Manage/Advise on the full SDLC of corporate L4 Repository implementations from URS creation through vendor selection, procurement and implementation for global serialisation operations.
Coding and Artwork
Site and Corporate Repositories
Coding and Artwork
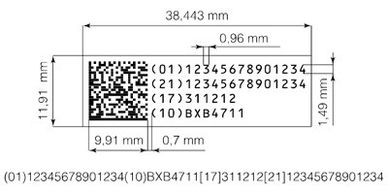
Creation of coding guidelines and technical specifications in line with various industry standards such as GS1 and EFPIA
Advise on pre-print, over print and artwork layout combinations based on manufacturers packaging equipment configurations and capabilities
Integration
Project Governance
Coding and Artwork
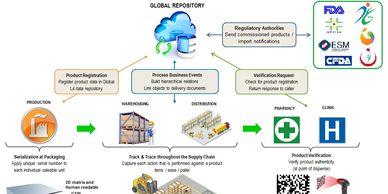
Support integration with 3PLs, distributors, wholesalers and other supply chain partners as necessary in order to meet the full obligations under the various consignment models and commercial agreements.
Support the contractual and technical on boarding with the various in market verification and reporting systems
Regulatory
Project Governance
Project Governance

Tracking and interpretation of evolving global serialisation regulatory requirements such as EU FMD, DQSA, Federal Law No. 425-FZ, SFDA circulars, ANVISA RDC 54 and CFDA decrees etc.
Project Governance
Project Governance
Project Governance

Creation of PM documentation in line with best practices to ensure successful roll out.
